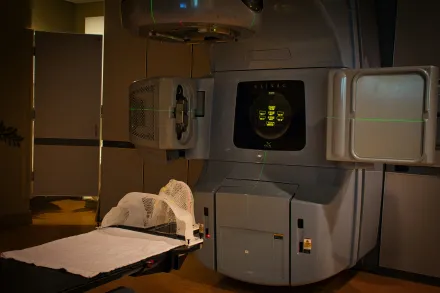
Radiation therapy has long been a cornerstone of cancer treatment and continues to evolve rapidly with advances in imaging, physics, and biology. The core idea behind radiation therapy is to use high energy particles or waves to damage the DNA of cancer cells, thereby limiting their ability to divide and survive. The process is not a blunt instrument but a carefully calibrated program that aims to maximize tumor control while preserving normal tissues as much as possible. The balance between efficacy and safety matters deeply for patients, because the success of therapy is influenced by precision, timing, and the integration of radiation with other modalities such as surgery and systemic therapies. Across many cancer types, radiation therapy serves as a primary curative option, a means to eradicate microscopic disease after surgery, a palliative approach to relieve symptoms, and a central component of multidisciplinary care that supports life extension and quality of life. In the modern clinic, radiation oncologists work with medical physicists, dosimetrists, radiographers, imaging specialists, and supportive care teams to translate a treatment plan into an actionable program of care that respects the biology of the tumor and the vulnerabilities of surrounding tissues. The practice of radiation therapy is thus both a technical field and a patient oriented discipline, grounded in scientific evidence, guided by patient values, and continually refined through research, clinical trials, and the cumulative experience of clinicians who dedicate themselves to compassionate, precise care.
Historical perspective and evolution
To understand the role of radiation therapy today, it helps to consider its historical arc, which begins with the discovery of X rays in the late nineteenth century and the realization that certain forms of radiation could alter living tissue. Early pioneers observed that radiation could shrink tumors, but they also learned that normal tissues were susceptible to injury, sometimes with serious consequences. Over decades, incremental advances in physics, imaging, and radiobiology gradually transformed a rough tool into a precise therapeutic modality. The introduction of cobalt therapy in the mid twentieth century offered a practical and accessible source of gamma rays, enabling standardized treatment delivery for many patients. As technology matured, linear accelerators replaced radioactive sources for external beam radiotherapy, expanding the versatility of beams, energies, and angles and enabling the shaping of dose distributions that spare critical structures. The emergence of three dimensional treatment planning, powered by computed tomography and later magnetic resonance imaging, allowed clinicians to visualize tumors in three dimensions and to craft dose clouds that conform tightly to tumor geometry. The late twentieth and early twenty-first centuries brought intensity modulated radiotherapy, image guided radiotherapy, and increasingly sophisticated planning algorithms that translate complex physics into patient specific plans. The field has continued to evolve with advances in motion management, adaptive strategies, and a growing appreciation for the interplay between radiation biology and clinical outcomes. Across this history, the emphasis has consistently been on transforming radiation from a blunt, tissue damaging force into a refined, targeted intervention that can be integrated with other therapies in a patient centered, multidisciplinary framework.
Fundamental principles of radiation therapy
At the heart of radiation therapy is the idea that energy deposition within tissue damages cellular components, particularly DNA, which can lead to cell death or permanent growth arrest. Cancer cells often have defects in DNA repair processes, a vulnerability that radiation therapy can exploit; normal cells, by contrast, typically possess more robust repair capabilities and a greater capacity for recovery when protected by careful planning. The therapeutic window arises from the differential response of tumor tissue and normal tissue to radiation, and the aim of treatment planning is to maximize tumor control probability while minimizing normal tissue complication probability. Dose is measured in units of gray, and the total dose is frequently delivered in multiple smaller fractions rather than as a single large dose. Fractionation allows normal tissues to repair sublethal damage between sessions while cancer cells, which may have impaired repair mechanisms, accumulate damage over time. The conceptual framework behind this approach includes radiobiologic models that describe how cells respond to different dose rates and fraction sizes, a consideration that informs fractionation schemes and overall treatment time. The precision of delivery is achieved through a combination of beam shaping, imaging guidance, and patient immobilization, all designed to align the radiation beams with the tumor while sparing adjacent organs and tissues that are critical to function and quality of life. In this sense the practice blends physics, biology, and clinical judgment in service of a cohesive treatment strategy that is tailored to the individual patient’s disease characteristics and anatomy.
Modern techniques and modalities
Today radiation therapy encompasses a suite of modalities designed to address a wide spectrum of cancers and clinical scenarios. External beam radiotherapy uses high energy X rays or particles delivered from outside the body and directed toward the tumor. Within this category, techniques such as intensity modulated radiotherapy adjust the intensity of the beam across the treatment field to conform the dose to complex tumor shapes, while volumetric modulated arc therapy delivers dose distributions as the machine rotates around the patient, reducing treatment times and improving conformity. Stereotactic approaches, including SBRT for body sites and SRS for intracranial targets, deliver very high doses in a small number of fractions with remarkable precision, often achieving tumor control rates comparable to surgery in selected settings. Proton therapy introduces a different physical property, the Bragg peak, which concentrates most of the energy at a specific depth, potentially reducing dose to overlying tissues and offering advantages for certain tumors near sensitive structures. Brachytherapy places radioactive sources directly within or adjacent to the tumor, delivering high local doses while sparing distant tissues, a strategy that remains essential for cancers of the cervix, prostate, and breast among others. Across these modalities, advances in imaging, motion management, and treatment planning have made it feasible to treat tumors with astonishing accuracy while keeping normal tissues within clinically acceptable exposure limits. The variety of options allows clinicians to choose strategies that align with tumor biology, anatomic considerations, patient preferences, and the goals of care, whether curative, palliative, or preparation for surgical intervention. In many cases, a patient may receive a combination of external beam radiotherapy and brachytherapy or a sequence that integrates with chemotherapy and targeted therapies, underscoring the collaborative nature of contemporary cancer care.
Treatment planning and imaging
The journey from diagnosis to delivered dose begins in planning CT simulations and often includes multimodal imaging such as MRI or PET to delineate tumor boundaries and identify critical structures. Contouring the tumor and surrounding organs at risk requires careful collaboration among radiation oncologists, radiologists, and other specialists. The plan is constructed in a planning system that calculates how dose will be distributed in three dimensions, enabling the planner to sculpt high dose regions to cover the tumor while constraining exposure to nearby organs. Dose constraints are established for sensitive tissues such as the spinal cord, lungs, liver, kidneys, and intestines, reflecting evidence about acceptable levels of risk for late radiation effects and early toxicities. The planning process also considers patient-specific factors, including prior treatments, ongoing medications, and comorbidities that could influence tolerance to treatment. Modern workflows emphasize image guidance during treatment, where in-room imaging verifies patient position and anatomy before each fraction. In some instances, adaptive radiotherapy is employed, in which the plan is adjusted during the treatment course in response to anatomical changes, such as tumor shrinkage or weight loss. This adaptive capability represents a convergence of imaging, software, and clinical judgment, enabling a dynamic approach to maintaining precision throughout the therapy course. The end result is a personalized, data informed plan that strives to maximize tumor control while protecting normal structures from unnecessary radiation exposure, a goal that sits at the core of patient safety and therapeutic effectiveness.
Clinical decision-making and indications
Decisions about radiation therapy are grounded in a careful assessment of tumor type, stage, location, biology, and the patient’s overall health and preferences. For some cancers, radiation therapy serves as a primary curative modality when tumors are localized and amenable to focused treatment; in others, it complements surgery by sterilizing microscopic residual disease that cannot be fully removed with scissors and sutures. In certain scenarios, radiation is used palliatively to relieve symptoms such as pain, bleeding, or obstruction, providing meaningful improvement in quality of life even when a cure is not possible. The choice of delivering external beam radiotherapy or brachytherapy, or a combination of modalities, depends on factors including tumor size, proximity to sensitive organs, and the desired balance between local control and potential side effects. Clinicians consider treatment timing in relation to surgery and systemic therapies, aiming to minimize overlapping toxicities while exploiting synergistic effects. The patient’s values and goals, including preferences about treatment duration, potential risks, and impact on daily living, weigh heavily in the final plan. In comprehensive cancer care, multidisciplinary tumor boards and shared decision-making processes ensure that radiation therapy is positioned within an integrated strategy that seeks to optimize outcomes, respect patient autonomy, and address practical considerations such as transportation, work, and family responsibilities. The result is a nuanced approach that balances scientific evidence with human factors to tailor therapy to the person facing cancer.
Radiobiology and fractionation
Radiobiology provides the conceptual framework for how different doses and fractionation schedules influence tumor and normal tissue responses. The linear quadratic model, a cornerstone of radiobiologic thinking, helps explain why smaller doses given over many fractions can reduce late normal tissue toxicity while preserving tumor control in many settings. The alpha/beta ratio, a parameter reflecting tissue sensitivity to dose per fraction, guides decisions about whether to use conventional fractionation, hypofractionation, or ultrahypofractionation in a given clinical scenario. Tumors with high proliferative rates or compromised DNA repair pathways may respond differently to fraction size, and treatment planning integrates these insights with practical constraints such as patient convenience and resource availability. Time is also an essential variable; extending the overall treatment course can lead to repopulation of tumor cells, whereas shortening the course may increase acute toxicity if not carefully managed. The biology of normal tissues is diverse, with some organs, like the spinal cord, showing relatively low tolerance to high doses per fraction, while others may be more forgiving. Understanding these nuances helps clinicians design fractionation schemes that optimize the therapeutic ratio. Ongoing research continues to refine radiobiologic models and to translate them into personalized schedules that account for tumor histology, genetics, microenvironment, and prior treatments, enriching the evidence base that supports clinical practice.
Benefits and risks
The benefits of radiation therapy can be substantial. For many cancers, radiation achieves high rates of local control and, in combination with other treatments, can contribute to long-term survival. In some settings, radiation also yields symptom relief without the need for invasive procedures, improving function and comfort for patients facing advanced disease. The risk profile of radiation therapy includes acute effects such as fatigue, skin irritation, and transient changes in organ function, as well as late effects that may emerge months or years after treatment, depending on the tissue involved and the total dose delivered. The risk of late toxicity underscores the importance of careful planning, image guidance, and dose constraints, particularly for organs with limited tolerance such as the optic nerve, spinal cord, and healthy brain tissue. The field emphasizes the principle of primum non nocere, striving to avoid harm while achieving therapeutic objectives. Patients may experience a range of side effects that reflect the tumor site and the specific tissues irradiated, and these effects are addressed with supportive care, proactive symptom management, and strategies to maintain nutrition, hydration, and physical activity during and after therapy. The balance between benefits and risks is not fixed; it evolves as new techniques reduce collateral exposure and as clinicians gain experience with how individual patients tolerate treatment, reinforcing the idea that radiation therapy is not a one size fits all intervention but a personalized instrument in the hands of a skilled care team.
Technology and quality of life
Advances in technology have significantly shaped the quality of life for patients undergoing radiation therapy. Enhanced imaging and motion management techniques reduce uncertainty and allow for precise targeting even when patients breathe or when tumors shift position slightly. The development of rapid treatment courses, such as hypofractionated regimens, can shorten the overall treatment duration, reducing the burden of daily visits and enabling patients to resume normal activities sooner. Even with shorter courses, the precision of delivery remains paramount to minimize acute and chronic toxicities, a goal supported by continuous verification of patient alignment and anatomy. The patient experience is also influenced by improvements in supportive care, including management of mucosal irritation, skin changes, and fatigue, as well as nutritional and psychosocial support. Interdisciplinary teams actively monitor for late effects, such as changes in organ function or secondary malignancies, and work to mitigate these risks through follow-up care, tailored rehabilitation, and ongoing health maintenance. In this way, technology and proactive care contribute to a broader aim: not only shrinking tumors but also preserving the physical and emotional well being of those who live with cancer, even long after treatment has ended.
Ethical and access considerations
Ethical considerations in radiation therapy include ensuring informed consent, balancing potential benefits with risks, and respecting patient autonomy in treatment choices. Access to cutting-edge modalities like intensity modulated and proton therapy varies across regions and health systems, with disparities shaped by resources, insurance coverage, and geographic location. These differences can influence the availability of specialized planning, image guided delivery, and adaptive strategies, which in turn affect outcomes and patient experience. Ethically, clinicians strive to provide care that aligns with evidence, but also acknowledges patient values, socioeconomic realities, and the reality of limited access in certain contexts. The ongoing challenge is to translate scientific advances into equitable care that reaches diverse populations, minimizes disparities, and supports shared decision making. Transparency about uncertainties, potential benefits, and the expected course of treatment helps patients navigate complex choices with confidence. As the field progresses, it remains essential to advocate for policies that expand access to high quality radiation therapy while ensuring safety, quality assurance, and patient safety at every step of the treatment journey.
Integration with other modalities
Radiation therapy rarely stands alone in modern cancer care. It often serves as a complementary component that enhances the effectiveness of surgery or systemic therapies. In many cases, preoperative radiation helps shrink tumors to enable less extensive surgical resections, potentially improving functional outcomes. Postoperative radiation targets residual microscopic disease, aiming to reduce the risk of recurrence and to extend disease free survival. In other situations, chemotherapy, targeted therapy, or immunotherapy are used in combination with radiation to exploit synergistic effects or to overcome resistance mechanisms. Timing and sequencing are critical, as simultaneous or sequential approaches each carry distinct toxicity profiles and clinical implications. The integration process requires careful coordination among surgeons, medical oncologists, radiation oncologists, and rehabilitation specialists, ensuring that treatment is coherent, patient centered, and optimized for maximal benefit. Across tumor types, multidisciplinary planning supports transitions between therapies, manages cumulative toxicities, and reinforces the patient’s overall treatment strategy, a reflection of how cancer care has evolved into a collaborative enterprise that values coordination as highly as technical proficiency.
Future directions and research
Looking forward, radiation therapy is poised to become even more precise, personalized, and adaptive. Research explores novel approaches such as ultra high dose rate delivery, sometimes referred to as FLASH therapy, which aims to spare normal tissues while maintaining tumor control, though clinical validation is ongoing. Image guided adaptive radiotherapy holds promise for adjusting plans in near real time to physiological changes, reducing margins, and enabling dose escalation when appropriate. Radiopharmaceutical therapies and targeted radionuclide approaches expand the spectrum of options by delivering radiation directly to cancer cells, offering complementary mechanisms to external beam strategies. The growing understanding of tumor microenvironments, hypoxia, and DNA repair pathways informs strategies to sensitize tumors or protect normal tissues. In the era of precision medicine, genetic and molecular profiling may influence how tumors respond to radiation, guiding individualized dose regimens. Data science, machine learning, and robust quality assurance frameworks continue to enhance planning accuracy, treatment verification, and outcome prediction. The horizons of radiation therapy thus combine cutting edge physics with evolving biology and patient centered care, aiming to improve outcomes while preserving the dignity, function, and hope of individuals facing cancer.
Radiation therapy remains a dynamic field that blends art and science to meet the diverse needs of patients. The collaboration between clinicians, physicists, imaging specialists, and supportive care teams creates a continuum of care that begins with a compassionate conversation about goals and ends with a carefully executed plan designed to transform the possibilities of treatment into tangible benefits. The importance of education for patients and families cannot be overstated, because understanding the purpose, process, and potential outcomes of radiation therapy empowers people to participate actively in decisions about their health. Throughout the patient journey, clinicians seek to minimize distress, maximize control, and preserve the ability to live with meaning and purpose, even as cancer treatment proceeds. The role of radiation therapy in cancer care is thus multifaceted and lasting, shaped by science and tempered by humanity, and its ongoing evolution reflects a shared commitment to extending life while respecting its quality and dignity.